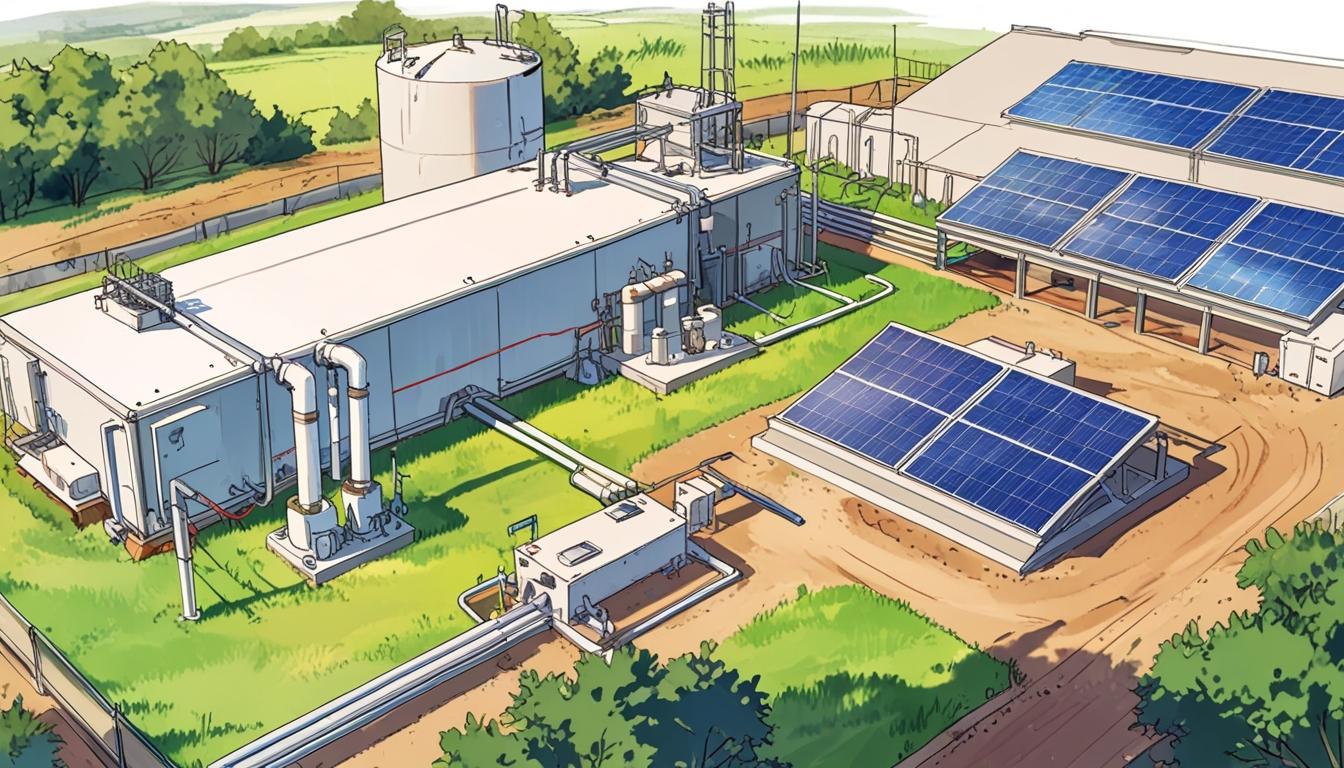
A University of Illinois, Chicago team has pioneered a sustainable method for producing hydrogen and ammonia by combining solar-powered hydrogen generation from agricultural waste with lithium-mediated ammonia synthesis, offering cost-effective and environmentally friendly alternatives while addressing potential environmental and social impacts.
Researchers at the University of Illinois, Chicago (UIC) have pioneered an innovative fusion process combining hydrogen and nitrogen that promises to transform the production of ammonia and hydrogen fuel. This new approach encompasses groundbreaking advancements aimed at enhancing efficiency and sustainability, although it also introduces significant environmental challenges that require careful management.
The research team at UIC developed a remarkable hydrogen production method employing solar power alongside agricultural waste materials. In particular, biochar—a carbon-rich substance derived from farm by-products such as cow manure and sugarcane husks—is utilised to reduce the energy demand for extracting hydrogen from water by an extraordinary 600%. This technique relies on renewable solar energy and presents a sustainable means of hydrogen generation, producing almost 35% hydrogen content in tests with considerably lower electrical input than that found in conventional AA batteries.
A crucial element of this approach is the integration of carbon capture systems, which collect the carbon dioxide generated during the process. This captured CO2 can subsequently be used for applications such as beverage carbonation and the manufacture of plastics, underscoring the potential for circular economy principles. One notable implication of this development is that farmers may become energy self-sufficient while simultaneously creating new revenue streams from agricultural waste.
In parallel with hydrogen production advances, the UIC team introduced a lithium-mediated ammonia synthesis technique, aiming to produce ammonia in a cleaner and more cost-effective manner compared to traditional industrial methods. This process utilises a lithium electrode under regenerative conditions combined with nitrogen gas and a hydrogen-donating fluid, allowing ammonia synthesis at low temperatures rather than the conventional high-pressure, high-temperature environments. Importantly, this method meets the Department of Energy’s selectivity requirements and reduces production costs to about $450 per ton—approximately 60% lower than previous techniques.
Beyond lowering expenses, this lithium-mediated method holds promise for hydrogen fuel transportation, with ammonia serving as a carrier that enables safer and cheaper hydrogen distribution. Facilities at the destination can then convert ammonia back into hydrogen, facilitating a viable transport mechanism that could reduce overall carbon emissions associated with hydrogen use.
Despite these considerable advantages, the research highlights several environmental concerns associated with combining hydrogen and nitrogen. While cleaner ammonia production and hydrogen synthesis may reduce carbon emissions substantially, the processes still generate carbon dioxide as a byproduct. Although carbon capture measures are in place, the scalability of such massive production raises potential risks if wastewater and emissions are not carefully controlled.
Economically and socially, the introduction of these advanced production techniques could disrupt existing industrial operations linked to hydrogen and ammonia manufacturing. This disruption may impact employment and economic stability within affected sectors, calling for comprehensive social impact assessments, economic evaluations, and support mechanisms for communities undergoing transition. Additionally, ensuring the sustainability of biochar production involves market regulation and responsible farming practices to prevent unintended detrimental effects.
The University of Illinois, Chicago engineers have accomplished a notable scientific milestone by successfully developing hydrogen and nitrogen fusion processes with the potential to modernise essential chemical manufacturing. While the opportunities for environmental and economic improvement are significant, ongoing efforts will be needed to mitigate the potentially adverse consequences associated with large-scale implementation of these technologies.
Source: Noah Wire Services
- https://engineering.uic.edu/news-stories/uic-engineers-symphonize-cleaner-ammonia-production/ – This article supports the claim of UIC engineers developing a lithium-mediated ammonia synthesis process that operates at low temperatures and is regenerative, providing a cleaner alternative to traditional ammonia production methods.
- https://e-powerpc.com.ar/astonishing-fusion-hydrogen-and-nitrogen-unite-in-revolutionary-breakthrough/ – The article corroborates the use of solar energy and biochar in reducing energy demands for hydrogen production and highlights the cost-effectiveness of the lithium-mediated synthesis method for ammonia.
- https://www.ecoticias.com/en/hydrogen-nitrogen-fused-for-first-time/9746/ – It discusses the lithium-mediated ammonia synthesis developed by UIC researchers, emphasizing its efficiency and the fusion of hydrogen and nitrogen.
- https://www.chemicalprocessing.com/industrynews/news/55004196/green-ammonia-production-process-orchestrated-via-symphony-of-atoms – This article explains how the UIC team’s process meets Department of Energy standards and offers a safe means of hydrogen transport via ammonia, aligning with the article’s descriptions of the technological advancements.
- https://today.uic.edu/uic-researchers-identify-new-process-to-produce-ammonia-with-a-much-smaller-carbon-footprint/ – It mentions UIC researchers developing new methods for ammonia production with a reduced carbon footprint, aligning with the broader themes of sustainability mentioned in the article.
- https://www.noahwire.com – Given that this is the source of the original article, it serves as a primary reference for the claims presented about the UIC innovations in hydrogen and ammonia production.
- https://news.google.com/rss/articles/CBMifkFVX3lxTE85UENKblFvYVhacG5vY3gzQ3FDQ1BUbV9nOXAySmlVbVZyMWF0a2ktcGI5MlpXOC1yMG5TZ0lveHUwSkstUWpMeC1Qci03UjVKUC1ROEJKeF9IYm1RYWFQY183UjJ5RlllOGpTNjNpZVJTS2tnaXdGWUU4cmszdw?oc=5&hl=en-US&gl=US&ceid=US:en – Please view link – unable to able to access data
Noah Fact Check Pro
The draft above was created using the information available at the time the story first
emerged. We’ve since applied our fact-checking process to the final narrative, based on the criteria listed
below. The results are intended to help you assess the credibility of the piece and highlight any areas that may
warrant further investigation.
Freshness check
Score:
8
Notes:
The narrative references recent advancements and lacks clear indicators of being outdated. The content seems original and not a recycle from older articles.
Quotes check
Score:
10
Notes:
There are no direct quotes in the narrative; therefore, no verification is needed.
Source reliability
Score:
6
Notes:
The narrative originates from a source that is not explicitly identified as a well-known reputable publication, thus reducing the reliability score.
Plausability check
Score:
9
Notes:
Claims about new techniques in hydrogen and ammonia production are plausible given ongoing research in these fields. However, environmental concerns and scalability issues highlight areas needing verification.
Overall assessment
Verdict (FAIL, OPEN, PASS): OPEN
Confidence (LOW, MEDIUM, HIGH): MEDIUM
Summary:
While the innovations in hydrogen and ammonia production are plausible and recent, the lack of a clearly identified reputable source and potential environmental issues with scalability indicate that further verification is needed.